For further information or Booking..
Creating Test Tube Babies: The IVF and ICSI Procedures
The process of creating a “test tube baby” through in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) involves several carefully planned steps. These procedures are designed to help individuals and couples experiencing fertility challenges achieve pregnancy.
Below are the seven key steps involved in the IVF process:
1. Ovarian Stimulation
On the second or third day of the menstrual cycle, blood tests are performed to measure sex hormone levels, which helps assess ovarian function. A transvaginal ultrasound is also conducted to evaluate the ovaries and count the number of developing follicles during that cycle.
Ovarian stimulation involves the administration of medications to stimulate the ovaries to produce multiple eggs. These medications are typically given as injectable drugs, and several stimulation protocols may be used depending on the patient’s condition.
At Phyathai Sriracha Hospital, a standardized stimulation protocol is commonly used, as it is both convenient and effective.
Stimulation begins on the second or third day of the menstrual cycle after hormone levels and ultrasound findings have been evaluated. The dosage of stimulation medication is individualized based on hormone levels, ultrasound results, and the age of the female partner. In general, two to three types of medications are used during ovarian stimulation.
The first medication is recombinant follicle-stimulating hormone (FSH), which promotes the growth of multiple follicles. This medication is typically administered for approximately five days.
The second medication is a gonadotropin-releasing hormone (GnRH) antagonist, which is used to prevent premature ovulation. This medication is especially important when multiple follicles are developing, as premature egg release could result in the loss of eggs before retrieval.
The usual duration of ovarian stimulation is 10–12 days. During this period, hormone levels are closely monitored, and ultrasound examinations are performed approximately three times to assess follicular growth. The timing of egg retrieval is determined based on follicle size. In general, follicles suitable for retrieval should measure approximately 18 mm or larger.
The final medication administered is human chorionic gonadotropin (hCG), which triggers final egg maturation. Ovulation typically occurs within 36 hours after the hCG injection, at which point the eggs are ready for retrieval.

2. Egg Retrieval
Egg retrieval is performed transvaginally using a thin needle attached to an ultrasound probe. The ultrasound probe is used to accurately locate the ovarian follicles during the aspiration procedure. The procedure is carried out under sedation and anesthesia to ensure patient comfort.
Using ultrasound guidance, the eggs are gently aspirated from the follicles. The entire procedure typically takes less than 30 minutes. The collected follicular fluid is then examined under a microscope to identify and isolate the eggs. Once retrieved, the eggs are placed in a culture medium and prepared for the fertilization process.

3. Sperm Collection and Preparation
Sperm preparation involves separating sperm cells from the seminal fluid to optimize them for fertilization. The male partner is advised to abstain from sexual intercourse for approximately 3–7 days prior to semen collection.
The semen sample is collected in a sterile container specifically designed for assisted reproductive techniques (ART). To maintain sperm viability, the sample should be delivered to the laboratory within 30 minutes after ejaculation. Delays in processing may reduce the sperm’s fertilization potential.
Once separated, the sperm are placed in a nutrient-rich culture medium to enhance motility and prepare them for the fertilization process.
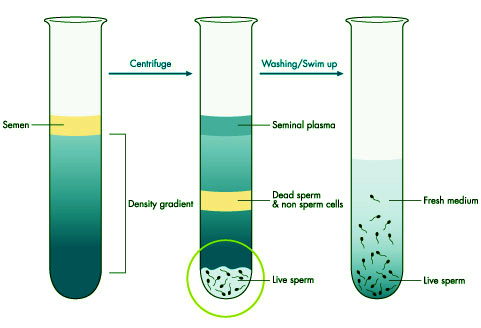
The process involves washing the sperm sample through a separation medium followed by centrifugation to remove debris and impurities. After preparation, the viable sperm are mixed with the eggs for fertilization.
4. Fertilization of Eggs with Sperm
In conventional in vitro fertilization (IVF), fertilization occurs by allowing sperm to swim freely and fertilize the eggs naturally in a laboratory environment. An appropriate number of viable sperm are added to the eggs and incubated for a specified period. After incubation, fertilization is assessed to confirm whether embryo development has begun.
This method may not be suitable for male partners with a low sperm count or poor sperm quality below standard reference values, as the risk of fertilization failure is higher. In such cases, intracytoplasmic sperm injection (ICSI) is recommended to improve fertilization outcomes.
5. Embryo Culture
The embryos are cultured in a specialized incubator under strictly controlled temperature and environmental conditions to support cell division and development. Beginning as a single cell, the embryos divide sequentially into two-cell, four-cell, and eight-cell stages.
Following early development, the embryos are carefully evaluated to determine whether they should be transferred to the uterus or further cultured to reach the blastocyst stage, depending on embryo quality and clinical considerations.
6. Embryo Transfer into the Uterus
Embryo transfer is a simple procedure similar to a routine gynecological examination. It is typically painless and does not require anesthesia. A thin catheter is gently inserted through the cervix to place the selected embryo(s) into the uterus.
In certain cases—such as when the female partner is unable to tolerate vaginal instrumentation or experiences significant anxiety—light sedation or anesthesia may be used to facilitate a smoother and more comfortable embryo transfer process.
To improve accuracy and success rates, ultrasound guidance is commonly used during embryo transfer. Ultrasound allows the physician to identify the optimal location within the uterine cavity for embryo placement, which has been shown to enhance implantation outcomes compared to transfers performed without ultrasound guidance.


7. Cryopreservation of Remaining Embryos
Cryopreservation of surplus embryos after embryo transfer is an effective method that significantly improves pregnancy outcomes compared with repeated ovarian stimulation cycles. With advances in cryopreservation techniques, embryos can be safely frozen and stored for several years and later transferred without the need for additional ovarian stimulation or egg retrieval.
In frozen embryo transfer (FET) cycles, the endometrium develops in a more natural and controlled hormonal environment compared with fresh transfer cycles following ovarian stimulation. During stimulated cycles, the endometrium may become excessively developed and less synchronized with embryo growth, which can negatively affect implantation.
As a result, fresh embryo transfer may not always be optimal, particularly in cycles with a strong ovarian response. Cryopreservation followed by frozen embryo transfer often provides higher implantation and pregnancy rates, especially in cases where multiple eggs are stimulated and multiple embryos are available.

There are several cryopreservation methods currently available. Phyathai Sriracha Hospital has developed its own vitrification technique and prepares specialized cryopreservation solutions in-house, without relying on imported products. The hospital has nearly a decade of experience in embryo cryopreservation using this method, achieving a post-warming embryo survival rate of over 95%. Cryopreserved embryos demonstrate developmental potential comparable to that of fresh embryos.
Based on these outcomes, embryo transfer can be performed either with fresh embryos immediately after fertilization or with cryopreserved embryos in subsequent frozen embryo transfer (FET) cycles. Cryopreservation often provides higher implantation and pregnancy rates, as embryos are transferred in a more physiologically optimal uterine environment using advanced preservation techniques.